
The picture above shows you that carbonic acid is H2CO3, it has 2H, 1C, and 3Os and how it is formed, umm the picture also shows a precipitation reaction btw, because calcium carbonate is insoluble and it will sort of precipitates out of solution by linking up with other calcium carbonate to form clumps.Īnyway, the key idea above for the baking soda reaction is that the real part of an acid that gives it acidic properties, which is the H+ ion is the one THAT REALLY REACTS at all, so the H+ ion reacts with the part of bicarbonate that is gives it basic properties which is actually the HCO3 to form voila! H2CO3 The carbonic acid decomposes to water and carbon dioxide gas. At pH values of between about 6.5 and 10. When water is added, the acid and bicarbonate combine to make a salt and carbonic acid. The equation for the reaction between carbon dioxide and water may be introduced for appropriate students. At pH values of less than about 6, mostly dissolved carbon dioxide and a small amount of carbonic acid exist in water. Baking powder is a mixture of acetic acid and baking soda. In themselves, they do not resolve the cementing problem.YEAH! acid and bases may sound like martians or other kind of aliens, but hey look at this, it is inolved in the baking of everyday’s good old bread!īaking soda is sodium bicarbonate.
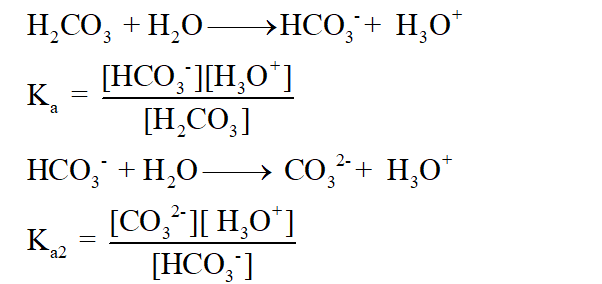
Acid-residue fertilizers assist in preventing cementation. Mithoron at 19:59 could you please give me the longer explanation user510 at 20:58 As Mithoron mentioned, H2CO3 is a unstable compound and was only isolated as a pure compound recently. It is an expensive product and should be included in a multiple-product approach.įeSO4 (Ironsul) x H2SO4 (free sulfuric acid) + CaCO3 (lime) gives FeSO4 (iron sulfate) + CaSO4 (gypsum). 1 Short answer is that youre right, but quite a bit can be told about carbonic acid and existence of H2CO3 molecule. Ironsul can be used in soils with or without lime, but it does work better in the presence of lime.
#Carbonic acid precipitate equation plus
Phosphoric acid (H2PO4) plus lime (CaCO3) gives calcium phosphate (CaH2PO4). Besides sulfuric acid, phosphoric acid can be used on lime-containing soils. S + Thiobacillus + warm and wet soil = H2SO4 (sulfuric acid) The reaction between sulfuric acid and lime to form gypsum takes minutes. dioxide, and when the carbon dioxide dissolves it forms carbonic acid.
#Carbonic acid precipitate equation free
Sulfuric acid reacts with the free lime (CaCO3) which is insoluble in water. Acid rain, also known as acid deposition, is any form of precipitation with. CO2 reacts with water to form carbonic acid (Equation 1).

Elemental sulfur (S) is converted to sulfuric acid (H2SO4) by Thiobacillus bacteria in warm, wet soil. Acid rain is defined as any form of wet precipitation - fog, dew, snow, hail or rain - which. In other words, taking calcium out by solubilizing it in a sulfate form. This is asking for trouble!! Lime or calciferous soils need to be treated with sulfur to form gypsum from the calcium already present in the soil. In other words, calcium is added to treat a high-calcium problem. In this section of the course we will consider the effect of carbon dioxide on water pH, the influence of solid phase calcium carbonate on solution composition and the implications of these reactions. Gypsum (CaSO4) is used by many to treat a lime (CaCO3) problem in soil. The simplest example of carbonates is the control which dissolved carbon dioxide has on water pH and buffering. Calcium in a sulfate form is soluble in water however, calcium sulfate (gypsum) can precipitate at high rates. Precipitation Reactions Here AB and CD are usually aqueous ionic compounds (or acids) consisting of aqueous ions (A + and B -, C + and D - ). Calcium in the presence of carbonic acid forms lime. (10.1) A B + C D A D + C B Reactions that can be classified as double replacements include precipitation reactions, neutralization reactions and gas forming reactions. This develops due to respiration in plant roots and due to decomposition of organic matter. Carbonic acid (H2CO3) is formed by the reaction of carbon dioxide (CO2) and water (H20). Calcium in a carbonate form is insoluble in water. The drainage system or rainwater may introduce carbonic acid to the system, which will react with the dissolved calcium and hydroxide ions (Equations 1 and 2). Calcium accumulates in soil in three ways: 1. Cementing is a problem that affects the top foot of the soil.Ĭalcium at 4500 ppm becomes a salt problem. is calcium greater or lesser than 4500, and 3. Three points to consider before selecting the proper amendment are: 1. This weak carbonic acid reacts with carbonates2 in rocks and silicates3 to form soluble calcium, magnesium and manganese ions4 and other minerals, the.


 0 kommentar(er)
0 kommentar(er)
